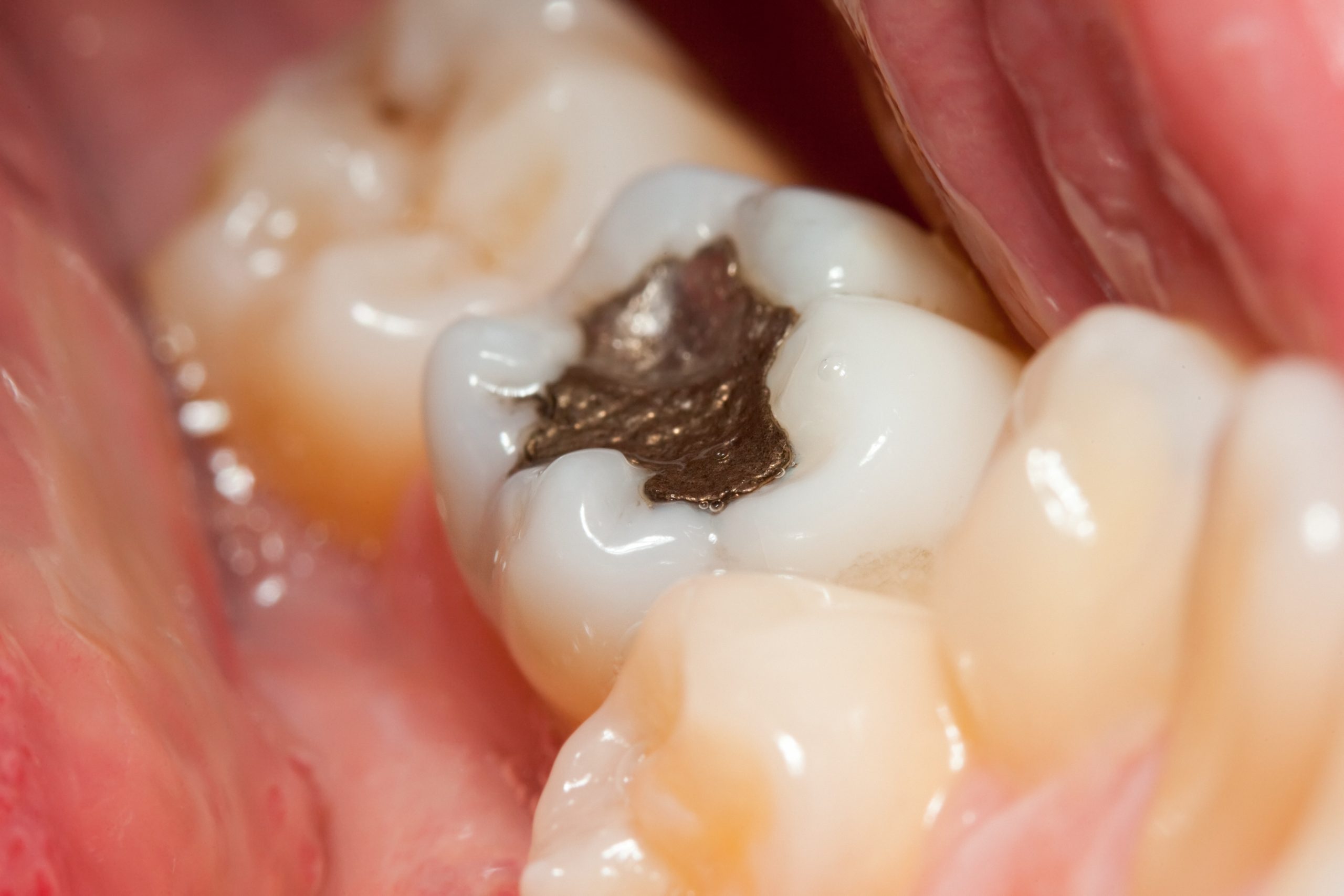
The United States Food and Drug Administration (FDA) has issued updated guidance on the use of dental amalgam, including a safety warning to health care providers and the public. The FDA notes it is recommending certain high-risk groups avoid getting dental amalgam whenever possible and appropriate.
The FDA states these groups may be at a greater risk for potential harmful health effects of mercury vapor released from the device. Therefore, the agency recommends the following groups avoid getting dental amalgams. These groups that may be at a greater risk for potential harmful health effects include:
- Pregnant women and their developing fetuses;
- Women who are planning to become pregnant;
- Nursing women and their newborns and infants;
- Children, especially those younger than six years of age;
- People with pre-existing neurological disease such as multiple sclerosis, Alzheimer’s disease or Parkinson’s disease
- People with impaired kidney function; and
- People with known heightened sensitivity (allergy) to mercury or other components of dental amalgam.
RELATED STORY:
To read the FDA announcement and summary of the updated guidance on the use of dental amalgam, use the first link below. To read the FDA safety warning to health care providers and the public on dental amalgam, use the second link below.
https://www.fda.gov/news-events/press-announcements/fda-issues-recommendations-certain-high-risk-groups-regarding-mercury-containing-dental-amalgam?utm_medium=email&utm_source=govdelivery (FDA announcement and summary of updated guidance on dental amalgam)
https://www.fda.gov/medical-devices/safety-communications/recommendations-about-use-dental-amalgam-certain-high-risk-populations-fda-safety-communication?utm_medium=email&utm_source=govdelivery (FDA safety warning to health care providers and the public on dental amalgam)