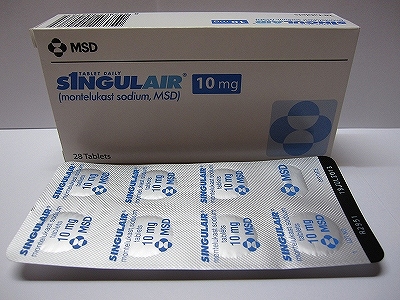
The popular asthma drug known as Montelukast or Singulair, which has been linked to cases of suicidal thoughts and depression in children, will now be sold with side effect warnings inside its packaging. (It is normally prescribed for children aged two to 14 with frequent intermittent, mild persistent or exercise-induced asthma.)
RELATED STORY:
And according to many parents, it’s about time.
“Vanessa Sellick’s son Harrison had been taking the drug since he was two years old and suffered serious behavioural changes. ‘Harrison was four years old when he started making comments about wanting to die, wishing that he was dead, that he was a piece of garbage — he had terrible self-loathing,’ Ms Sellick said. ‘It was just devastating at the time.'”1
Since then, she’s been working to for better warnings about the drug and for doctors to have education about its risks.
And now, following a review of its safety, the Therapeutic Goods Administration (TGA) “has decided that information about the drug’s side effects should be included in boxes.”2They have also called for “health authorities to include warnings in guidelines for health professionals, and called for better monitoring of how many kids might be suffering serious side effects.”3
While this is a step in the right direction, Sellick wants the government to do more; because this medication has been linked to death by suicide, she wants warning labels attached.
If you are in Australia and you have questions or need help, call one of the numbers below:
- Lifeline 13 11 14
- Kids Helpline 1800 551 800
- Beyond Blue 1300 22 46 36
- Headspace 1800 650 890
Between 2000 and December of 2017, there were 167 adverse neuro-psychiatric side effects in children and adolescents taking the drug.
RELATED STORY:
Following the death of a teenager taking the drug in 2008, health authorities in the US have been investigating its safety. Cody Miller, a 15-year-old, had only been taking the drug for 17 days when he took his own life.
The company behind Singulair, Merck, Sharp and Dohme (MSD), has said that the drug has been used safely by tens of millions of people around the world and that “Consumer medicine information is made available to patients via the TGA website or the MSD website.”4
SOURCE: